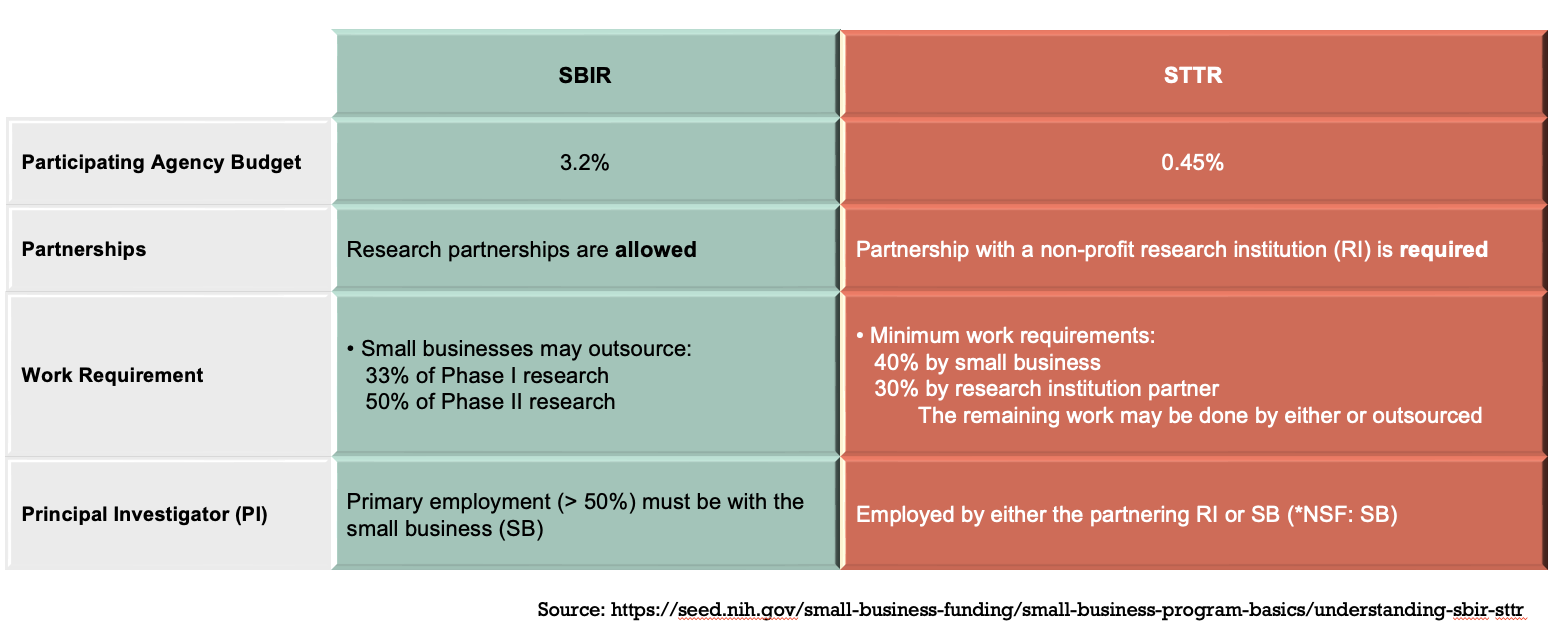
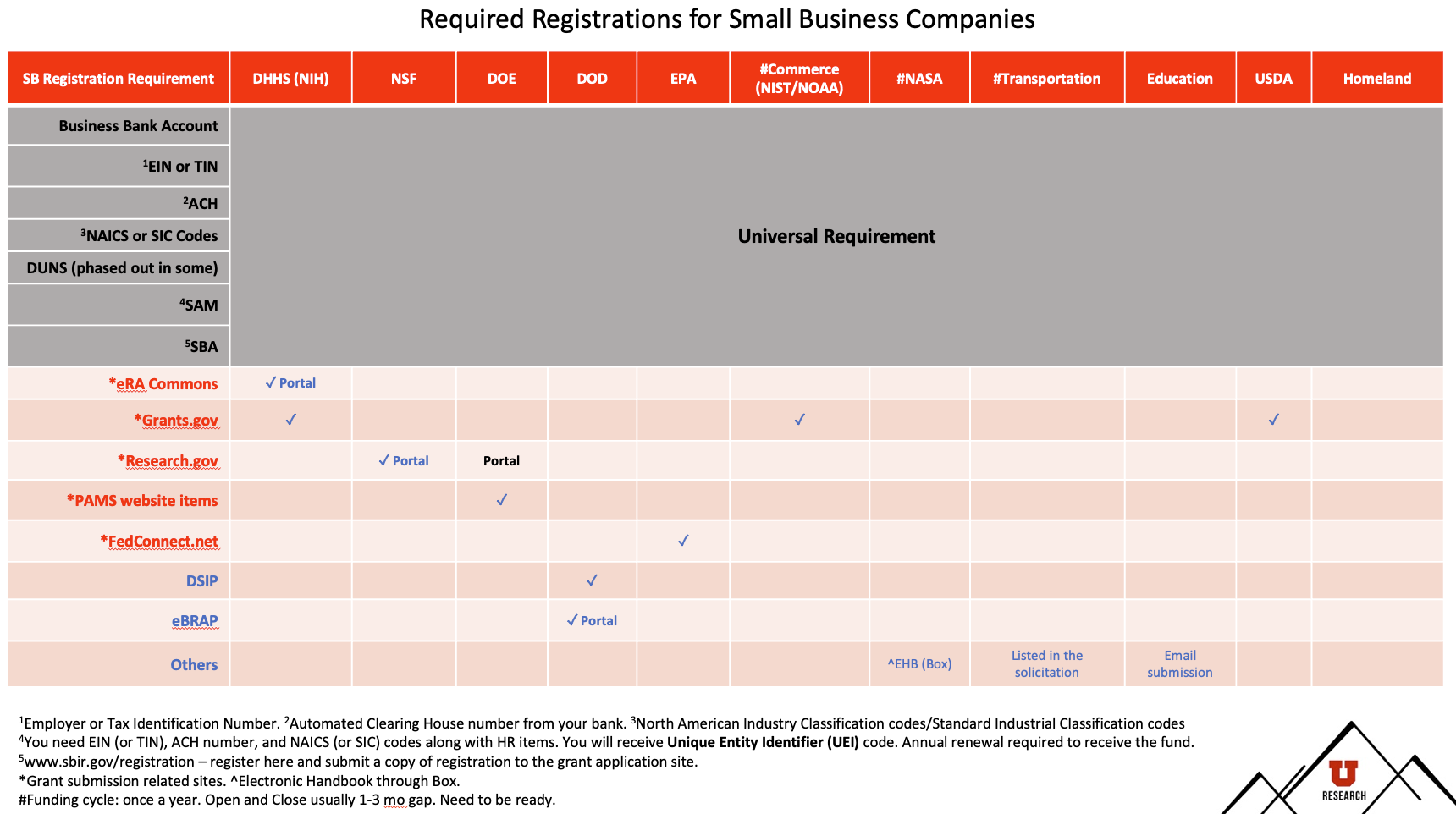
SBIR/STTR Programs from 11 federal agencies fund
small business companies' (SBCs) innovative research with commercialization potentials in various topics.
Summary of Topics and Funding Agencies
National Institutes of Health
Learn More
National Science Foundation
Learn More
Department of Energy
Learn More
*Direct Phase II avaiavle (pilot program). Not available for STTR. CDC/FDA do not participate
TABA: Techincal and Business Assistance Funding (market research, development of regulatory/manufacturing plans, IP)
Source: sbir.gov (2023)
SBIR/STTR programs are multiphase funding
Phase
Goal
Funding
I
Proof of Concept/Testing feasibility
Up to $295K (for 0.5 - 2 yrs)
II
Validation and further development
Up to $1.8M (for 2 - 3 yrs)
II+ or III
Commercialization process
lib (bridge grant), CRP, etc.